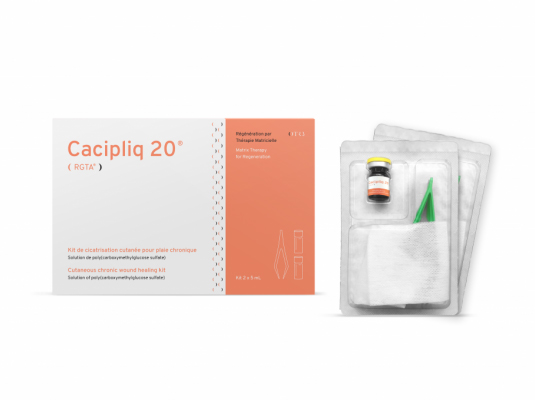
Further information on Cacipliq 20®
Cacipliq 20® is based on the ReGeneraTing Agents (RGTA®) technology, which consists of modified biodegradable sugars.
It protects extracellular matrix proteins, helping to restore their original structure and preserve the natural cellular microenvironment of the wound, as well as the endogenous factors required for tissue regeneration.
Supplied as a sterile solution for easy application twice weekly, Cacipliq 20® promotes rapid and effective wound healing. It is a unique, easy-to-use tissue regeneration solution proven to accelerate wound healing, including in chronic, non-healing ulcers such as those associated with diabetes.
For chronic ulcers showing no tendency to heal after 6 months of conventional care, or untreated ulcers after 12 months including :
- Bedsores
- Ulcers following peripheral arterial disease (such as Leriche & Fontaine stage 4)
- Diabetic ulcers (including amputation)
- Sterile solution for external use.
- Spray: box containing a 7.5 ml bottle: around 50 pushes, 3K® pump guaranteeing sterility for 6 months.
- Kit: 5 ml vial with sterile compress, 2 per box.
- Preservative free.
- Reduced healing time
- Excellent product safety history
- Easy to use